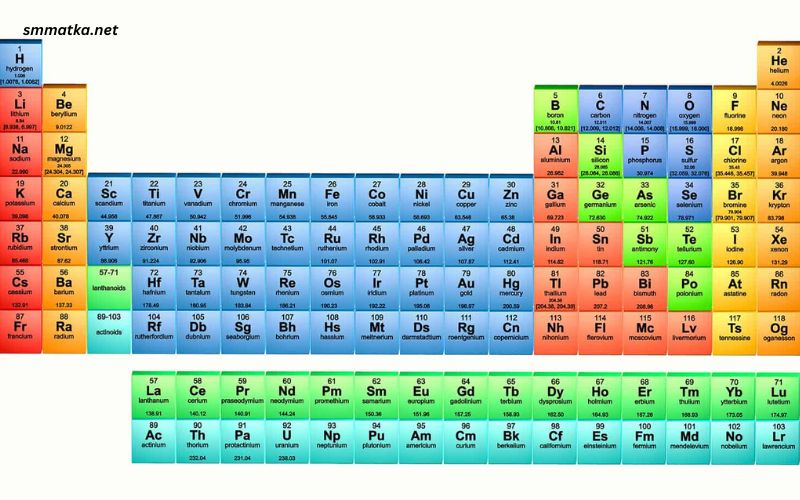
The periodic table is one of the most iconic symbols of chemistry, a comprehensive chart that organizes the elements in a way that reveals the underlying principles of their chemical behavior. Its invention revolutionized our understanding of chemical properties and relationships, enabling scientists to predict the behavior of elements and compounds with remarkable accuracy. This article delves into the history, structure, and significance of the periodic table, exploring its role in modern science and education.
The History of the Periodic Table
The periodic table’s history is a story of scientific curiosity and discovery. Before its creation, chemists struggled to organize the known elements in a coherent manner. By the mid-19th century, around 60 elements had been discovered, but their properties seemed disparate and unconnected.
The first significant step towards a systematic arrangement came from Johann Wolfgang Döbereiner in the early 1820s, who noticed that certain groups of three elements (triads) had similar properties. He observed that the atomic weight of the middle element in these triads was roughly the average of the other two. Although his triads were a limited observation, they hinted at a deeper relationship among elements.
In 1862, Alexandre-Émile Béguyer de Chancourtois, a French geologist, arranged the elements in a spiral on a cylinder based on increasing atomic weights, recognizing that elements with similar properties appeared at regular intervals. This “telluric screw” was a precursor to the modern periodic table, but it did not gain much attention at the time.
The breakthrough came with Dmitri Mendeleev, a Russian chemist who, in 1869, published his first periodic table. Mendeleev’s genius lay in his ability to recognize patterns and make bold predictions. He arranged the 63 known elements by increasing atomic weight, leaving gaps where no known element fit and predicting the properties of elements that would later be discovered, such as gallium and germanium. Mendeleev’s periodic law stated that the properties of elements are a periodic function of their atomic weights, a concept that proved remarkably accurate.
Mendeleev’s table was refined over the following decades. The discovery of noble gases by Sir William Ramsay in the 1890s added a new group to the table. The concept of atomic number, introduced by Henry Moseley in 1913, provided a more accurate basis for the arrangement of elements. Moseley’s work showed that the atomic number, rather than atomic weight, was the fundamental property that determined an element’s position in the periodic table.
Structure of the Periodic Table
The modern periodic table is structured around the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. This means that elements with similar properties recur at regular intervals when arranged by increasing atomic number. The table is divided into rows (periods) and columns (groups or families), each with its own significance.
Periods
There are seven periods in the periodic table, each corresponding to the number of electron shells in the atoms of the elements in that period. As you move from left to right across a period, the atomic number increases, and the elements gradually change from metals to nonmetals. This change is due to the increasing number of protons in the nucleus and the corresponding increase in the number of electrons in the outer shell, which affects the element’s chemical properties.
For example, in the second period, lithium (Li) is a highly reactive metal, while neon (Ne) is an inert noble gas. This trend is consistent across all periods: metals are found on the left, metalloids in the middle, and nonmetals on the right.
Groups
The vertical columns in the periodic table are called groups or families. Elements in the same group share similar chemical properties because they have the same number of valence electrons. There are 18 groups in the modern periodic table, each with its own set of characteristics.
- Group 1: Alkali Metals – These elements, including lithium (Li), sodium (Na), and potassium (K), are highly reactive metals with one valence electron. They react vigorously with water to form alkaline solutions.
- Group 2: Alkaline Earth Metals – This group includes beryllium (Be), magnesium (Mg), and calcium (Ca). They are less reactive than alkali metals and have two valence electrons.
- Groups 3-12: Transition Metals – These elements, such as iron (Fe), copper (Cu), and gold (Au), are characterized by their ability to form variable oxidation states and complex ions. They are typically hard, dense metals with high melting points.
- Group 13: Boron Group – This group contains elements like boron (B), aluminum (Al), and gallium (Ga). They have three valence electrons and display a mix of metallic and nonmetallic properties.
- Group 14: Carbon Group – Including carbon (C), silicon (Si), and tin (Sn), these elements have four valence electrons. Carbon is a nonmetal, silicon and germanium are metalloids, and tin and lead are metals.
- Group 15: Nitrogen Group – This group, which includes nitrogen (N), phosphorus (P), and arsenic (As), has five valence electrons. They display a wide range of physical and chemical properties.
- Group 16: Oxygen Group – Also known as the chalcogens, this group contains oxygen (O), sulfur (S), and selenium (Se). They have six valence electrons and are typically reactive nonmetals.
- Group 17: Halogens – These elements, including fluorine (F), chlorine (Cl), and iodine (I), are highly reactive nonmetals with seven valence electrons. They readily form salts with metals.
- Group 18: Noble Gases – This group contains helium (He), neon (Ne), and argon (Ar). They are inert gases with a full outer electron shell, making them extremely stable and unreactive.
The Significance of the Periodic Table
The periodic table is more than just a tool for organizing elements; it is a powerful framework for understanding chemical behavior and predicting the properties of elements and compounds. Its significance extends to various fields of science and industry.
Predicting Chemical Behavior
The periodic table allows chemists to predict the behavior of elements and their compounds. By understanding the trends and patterns within the table, scientists can anticipate how elements will react with one another. For example, knowing that elements in Group 1 are highly reactive metals that form +1 ions, chemists can predict that sodium (Na) will readily react with chlorine (Cl) to form sodium chloride (NaCl), a common salt.
Identifying Unknown Elements
The periodic table has been instrumental in the discovery of new elements. Mendeleev’s predictions of missing elements and their properties led to the discovery of gallium (Ga), germanium (Ge), and scandium (Sc). Even today, scientists use the periodic table to guide their search for new elements, particularly in the realm of synthetic elements created in laboratories.
Understanding Atomic Structure
The periodic table reflects the arrangement of electrons in atoms, providing insight into atomic structure and bonding. The position of an element in the table reveals the number of valence electrons, which determines how it interacts with other elements. This understanding is crucial for fields such as quantum chemistry and materials science.
Applications in Industry and Technology
The periodic table has numerous practical applications in industry and technology. Elements are used in everything from electronics and energy production to medicine and manufacturing. For example, transition metals like copper (Cu) and silicon (Si) are essential for the production of electronic devices, while rare earth elements are critical for renewable energy technologies like wind turbines and electric vehicles.
Modern Developments
The periodic table continues to evolve as new elements are discovered and our understanding of atomic structure deepens. In recent decades, scientists have synthesized elements beyond uranium (element 92), known as transuranium elements. These elements, such as neptunium (Np), plutonium (Pu), and the superheavy elements like flerovium (Fl) and livermorium (Lv), are typically unstable and exist only briefly in laboratory conditions.
Advances in technology and experimental techniques have also led to the exploration of the periodic table’s theoretical limits. Researchers are investigating the possibility of an “island of stability” where superheavy elements might have relatively longer half-lives, offering new insights into nuclear physics and chemistry.
Conclusion
The periodic table is a fundamental tool in chemistry, encapsulating the principles that govern the behavior of elements and their compounds. Its development marked a turning point in scientific history, providing a framework for organizing the known elements and predicting the properties of those yet to be discovered. Today, the periodic table remains a vital resource for scientists, educators, and industry professionals, continually expanding our understanding of the natural world. As we continue to explore the boundaries of chemical knowledge, the periodic table will undoubtedly remain a cornerstone of scientific inquiry and discovery.